Chaos to Compliance
In the high-stakes world of medical device manufacturing, a brilliant product is not enough. You can build a system that detects cancer cells with unprecedented accuracy, but if you cannot prove consistent, validated performance to the FDA, you do not have a product—you have an expensive paperweight.
We recently partnered with a diagnostic innovator facing this exact challenge. They had developed a breakthrough “partially automated” cytology platform that bridged the gap between manual lab work and full automation. The engineering was sound. The science was validated.
But they faced a critical hurdle: The Documentation Fog.
Because the device relied on human operators to achieve machine-level precision, the regulatory burden was immense. How do you prove to the FDA that a manual process will yield the exact same “monolayer slide” every single time, across different labs and different operators?
The answer wasn’t just in the engineering. It was in the User Manuals, the Compliance Narrative, and the Technical Storytelling.
Project at a Glance: NexGene Diagnostics
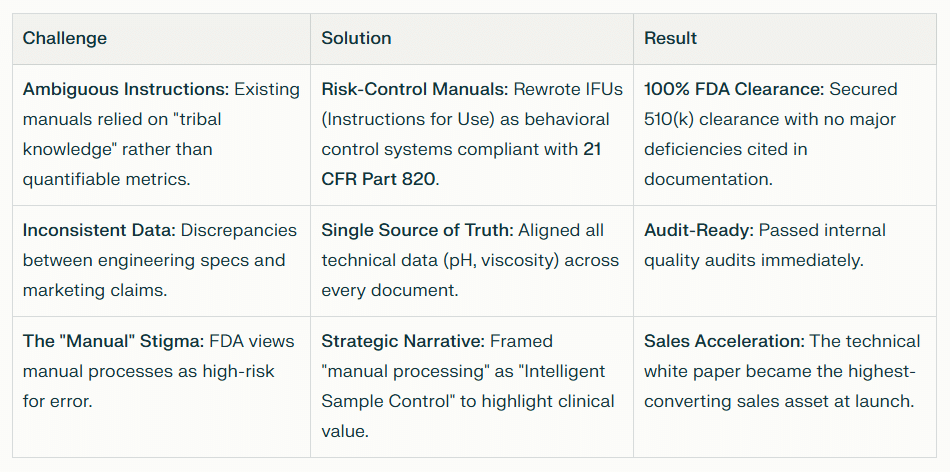
The Fix: Turning “Steps” into “Controls”
When we audited the project, we found the “Chaos” wasn’t in the hardware—it was in the information architecture. The existing documentation treated critical tasks as suggestions rather than mandates.
To bridge the gap from Chaos to Compliance, we implemented a three-tiered documentation strategy:
1. The User Manual as a Validation Tool
Standard manuals just list steps (e.g., “Mix the reagent”). For FDA submission, this is insufficient. We rewrote the user documentation to function as a behavioral control.
Precision Language: We replaced vague instructions with quantifiable metrics. “Mix well” became “Invert the vial 5 times to ensure viscosity specifications are met.”
Visual Validation: We integrated diagrams that showed users exactly what “success” looked like (a true monolayer) and what “failure” looked like (obscuring artifacts).
The Result: The manual didn’t just teach usage; it enforced the Quality by Design (QbD) principles the FDA demands.
2. The Compliance Narrative
A 510(k) submission is essentially a legal argument. You must prove “Substantial Equivalence” to a predicate device while highlighting your innovation. We crafted a narrative that turned the device’s potential weakness—its “manual” aspect—into its greatest strength.
We documented how the lack of full automation actually allowed for “Intelligent Sample Processing”—a feature that reduces glandular cell loss. This wasn’t marketing fluff; it was a technical argument backed by data, positioned perfectly for a regulatory reviewer.
3. The “Single Source of Truth”
Inconsistencies kill submissions. A marketing white paper cannot claim “100% cell retention” if the validation data shows 98%. We aligned every piece of collateral—from the Instruction for Use (IFU) to the all of the supporting documentation—to ensure specific technical values were identical.
The Lesson for Your Product
Whether you are building a SaaS platform, an aerospace component, or a medical device, the principle holds true: Ambiguity is risk.
Great documentation does more than describe your product—it validates it. It turns the chaos of “how does this work?” into the compliance of “this works exactly as intended, every time.”
Innovation Meets Regulation
NexGene developed VisiLayer®, a breakthrough “partially automated” cytology reagent system. While the technology offered superior glandular cell retention compared to fully automated competitors, its reliance on manual processing created a significant regulatory hurdle. To achieve FDA 510(k) clearance, NexGene needed to prove that their manual process was not just “flexible,” but controllable, repeatable, and validated. What we provided:
- White Papers
- Case Studies
- Product Manuals
- Installation Manuals
- Instruction Manuals
- User Manuals
- Training Manuals
- Regulatory Documentation
- Compliance Documentation
- Documentation for Medical Device Submissions
